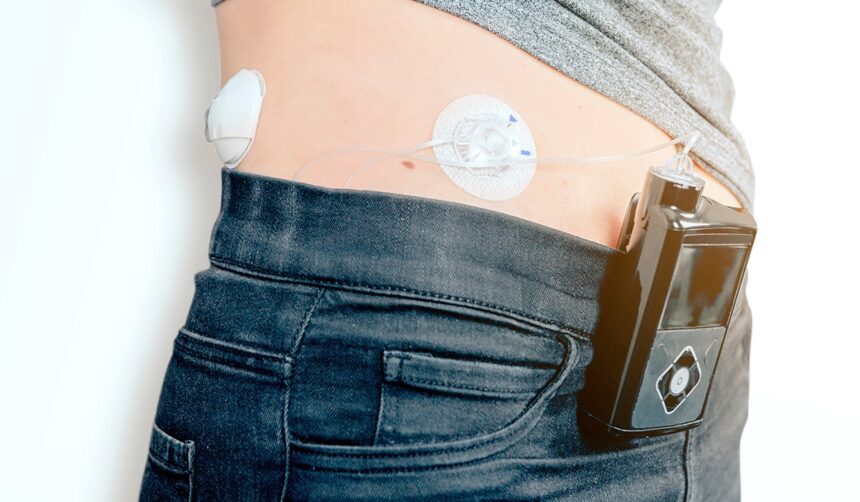
Automated insulin delivery systems, also called artificial pancreas, can improve glycemic outcomes and make daily self-care easier for people with type 1 diabetes.
Automated insulin delivery (AID) systems, also called artificial pancreas, help people with type 1 diabetes automate tasks such as monitoring their blood glucose and taking insulin. AID systems improve glycemic outcomes and quality of life for many people, but their high cost and other barriers prevent more widespread use. Sue Brown, MD, professor of endocrinology at the University of Virginia School of Medicine, describes how AID systems can help patients manage diabetes and what’s ahead for this innovative technology.
Q: What is an artificial pancreas?
A: Many people have used the term “artificial pancreas,” but my preferred term is “automated insulin delivery,” or AID. You may also see the term “closed-loop system.”
These systems automatically determine insulin requirements and adjust insulin delivery to meet the current needs of a person with type 1 diabetes. Most AID systems do this about every 5 minutes to keep people in their blood glucose target range as much as possible. These systems also reduce the risk of hyperglycemia and hypoglycemia.
These systems have three components that work together and communicate using Bluetooth technology
- a continuous glucose monitor (CGM) that measures the person’s blood glucose level
- an insulin pump that delivers insulin to the person’s body
- an algorithm that collects data from the CGM, calculates the person’s current insulin needs, and signals the pump to change or maintain insulin delivery
AID systems use current and historical information about insulin delivery, blood glucose targets, and actual blood glucose values. All of that information goes into calculations the system performs to determine how much insulin is required in the moment.
There are many systems with different configurations of devices and algorithms. This year alone, the U.S. Food and Drug Administration (FDA) has approved three new algorithms for use in AID systems. Most systems are for people age 6 and older with type 1 diabetes, but the FDA has approved at least one system for children as young as 2. Artificial pancreas systems have been studied in people with type 2 diabetes who need insulin, but they are not yet FDA-approved for this population.
Q: Why is this technology critical for people with type 1 diabetes? How can it improve diabetes management compared with CGMs or insulin pumps alone?
A: Many, if not most, people with type 1 diabetes do not meet the American Diabetes Association (ADA) goals for glycemic outcomes. This is especially true for adolescents and young adults. It is clear that different strategies are needed. AID is one such solution to improve outcomes.
We have lots of data showing that AID systems—regardless of the specific algorithm or configuration—consistently help people meet glycemic goals in clinical trials, as well as in real life. That means increasing time in range by about 2.5 hours per day on average, lowering hemoglobin A1C levels by 0.3% to 0.5%, and decreasing the risk of hypoglycemia. Of course, the exact numbers vary by individual. Notably, people see the most benefit from these systems overnight. This is a vulnerable time period for many people with type 1 diabetes because of the risk of hypoglycemia while sleeping.
The pivotal clinical trials compared AID systems to usual care. In some cases, participants used an insulin pump or CGM without an algorithm.
The beauty of AID is that it further automates the process of daily self-care and improves glycemic outcomes at the same time. This often means a better quality of life. Studies show that patients with type 1 diabetes and their caregivers may experience reduced anxiety and distress. The systems also may reduce fear of hypoglycemia among parents of children with type 1 diabetes, leading to better sleep.
Q: Which patients can benefit most from an AID system?
A: There’s yet to be a population with type 1 diabetes that hasn’t benefited when using AID. There have been studies in very young children (ages 2 to 5 years), older children, adolescents, and adults. Across those age ranges, we’ve seen a significant improvement in glycemic measures such as A1C level and time in target range for glucose. These improvements occurred in people of different incomes and racial and ethnic groups.
That doesn’t mean everyone in a clinical trial had the same outcomes. Often, participants who started out with very high A1C levels or lower time in range had the biggest improvements. But they didn’t necessarily attain as low an A1C or as high a time in range as those who started out at a lower A1C or higher time in range.
Among age groups, AID systems especially help adolescents with higher A1C levels and lower time in range. These automated systems can compensate when someone might miss a meal-time insulin bolus, for example. AID can decrease the burden of making clinical management decisions every day.
Q: What are the limits of AID systems?
A: The current FDA-approved devices perform better if users provide some sort of meal announcement. However, the person or their caregiver doesn’t have to know how to count carbs to use these systems. Instead, they can input a fixed amount of carbs for a meal-time insulin bolus as one method to make these systems easier to use. If they did not bolus for a meal, the AID systems can compensate to some degree. One of the newer systems decreases the burden still more by allowing users to indicate if they’re eating a usual meal or one that is bigger or smaller.
AID systems do not perform as well for exercise. There’s still quite an effort to manage the impact of exercise on blood glucose levels and resulting insulin needs. The systems often have a setting that can be adjusted to account for exercise, but this area is still a challenge.
In terms of the system components, users must understand that the insulin pump infusion set—the thin plastic tube that connects the insulin pump to the person’s body—can fail and how to detect that. They must also know when a CGM may not be accurate and how to handle that. Health care professionals can teach patients these skills along with how to use the AID system.
In a perfect world, we’d love an artificial pancreas that requires less management, where you can “set it and forget it,” but we’re not quite there yet.
Q: What percentage of people with type 1 diabetes use AID? What barriers prevent wider use of this technology?
A: We don’t know what percentage of people with type 1 diabetes use AID. We do know that the use of diabetes care technology, particularly CGMs, has risen significantly.
There are many factors to consider in deciding if AID is right for a patient. One of them is whether a person wants to wear an on-body device or not.
If a patient does want to get an AID system, cost and access are looming issues. The cost of AID systems varies but can be several thousand dollars. They are usually covered by insurance, but there are all kinds of variations in coverage. Sometimes the pump is covered but the CGM is not. I often write letters of appeal for patients whose health plans have denied coverage. Some manufacturers offer financial assistance programs. Still, the cost can put this technology out of reach for some patients. On top of that are the ongoing costs of insulin and supplies for CGMs and insulin pumps.
It appears that there are socioeconomic and racial and ethnic disparities in the use of diabetes technologies. Compared with non-Hispanic White patients, patients from other racial and ethnic groups are less likely to be on either a CGM or an insulin pump. I don’t know yet if that applies to AID systems. There are likely many reasons for these disparities, and I do want to highlight the issue of potential bias from health care professionals. By that I mean that patients who could benefit may not be offered an AID device or given assistance or encouraged to obtain or manage these systems, which is an important barrier that needs to be addressed.
Q: How can health care professionals learn about artificial pancreas systems and train patients to use them?
A: The first step is to keep abreast of changing technology. The various AID systems have their differences. Health care professionals must understand them to help patients select and use AID properly, according to clinical guidelines for initiating artificial pancreas systems, which were developed by an international panel. These guidelines include recommendations for training and education.
Technical training in AID should occur alongside education about overall diabetes management. Some may prefer to start with a CGM or insulin pump first, but I find that many who have never used diabetes technology can jump right into AID use.
Manufacturers of artificial pancreas systems also provide in-person and online training for health care professionals. Professional organizations also offer training. The PANTHER program run by the University of Colorado’s Barbara Davis Center for Diabetes has device fact sheets, a device-comparison chart, and more on its website.
Q: What is the future of artificial pancreas research? What gaps in knowledge remain? What technical improvements can be made?
A: There are a lot of persistent challenges in AID research. As I mentioned, cost and access are barriers. Another issue is managing postprandial hyperglycemia, or high blood glucose that occurs after meals. That’s related in part to how subcutaneous insulin acts inside the body, which is quite slow. Researchers are trying to see if some medications used for type 2 diabetes, such as GLP-1 receptor agonists, could be add-ons that help with postprandial hyperglycemia.
Also, exercise has a lot of complex effects on glycemia. There are a lot of reasons for that. One is that subcutaneous insulin action in the body is prolonged and doesn’t turn off quickly enough when needed during exercise. We need ways for AID systems to better regulate insulin to mitigate the risk of hypoglycemia after exercise.
Patients still have a lot of interactions with the AID system. Several research groups are working to lower this burden by creating algorithms that either do not require or minimize the need for insulin boluses to be given for corrections or meals.
Other researchers are looking at insulins with different actions in the body or different methods of insulin delivery. Dual-hormone systems that administer insulin along with other hormones such as glucagon, which is used to treat hypoglycemia, are also being studied.
Finally, we need larger and longer-term trials of AID systems to better understand the systems’ ability to provide long-term benefits for metabolic control.
Do you offer artificial pancreas systems to your patients? Share below in the comments.